This fundamental concept, validated through a series of rigorous studies, has now been successfully applied to the challenging realm of therapeutic cancer vaccines, specifically targeting human papillomavirus (HPV)-driven tumors. In their latest published research, the Northwestern team demonstrated that merely adjusting the precise orientation and spatial position of a single cancer-targeting peptide within a vaccine construct dramatically amplified the immune system’s capacity to recognize and attack malignant cells. This seemingly subtle structural modification unlocked a far more potent anti-tumor response, underscoring the profound implications of nanoscale precision in immunotherapy.
The findings of this pivotal study were unveiled on February 11 in the esteemed scientific journal Science Advances, signifying its importance to the broader scientific community.
Pioneering the Spherical Nucleic Acid Vaccine Platform
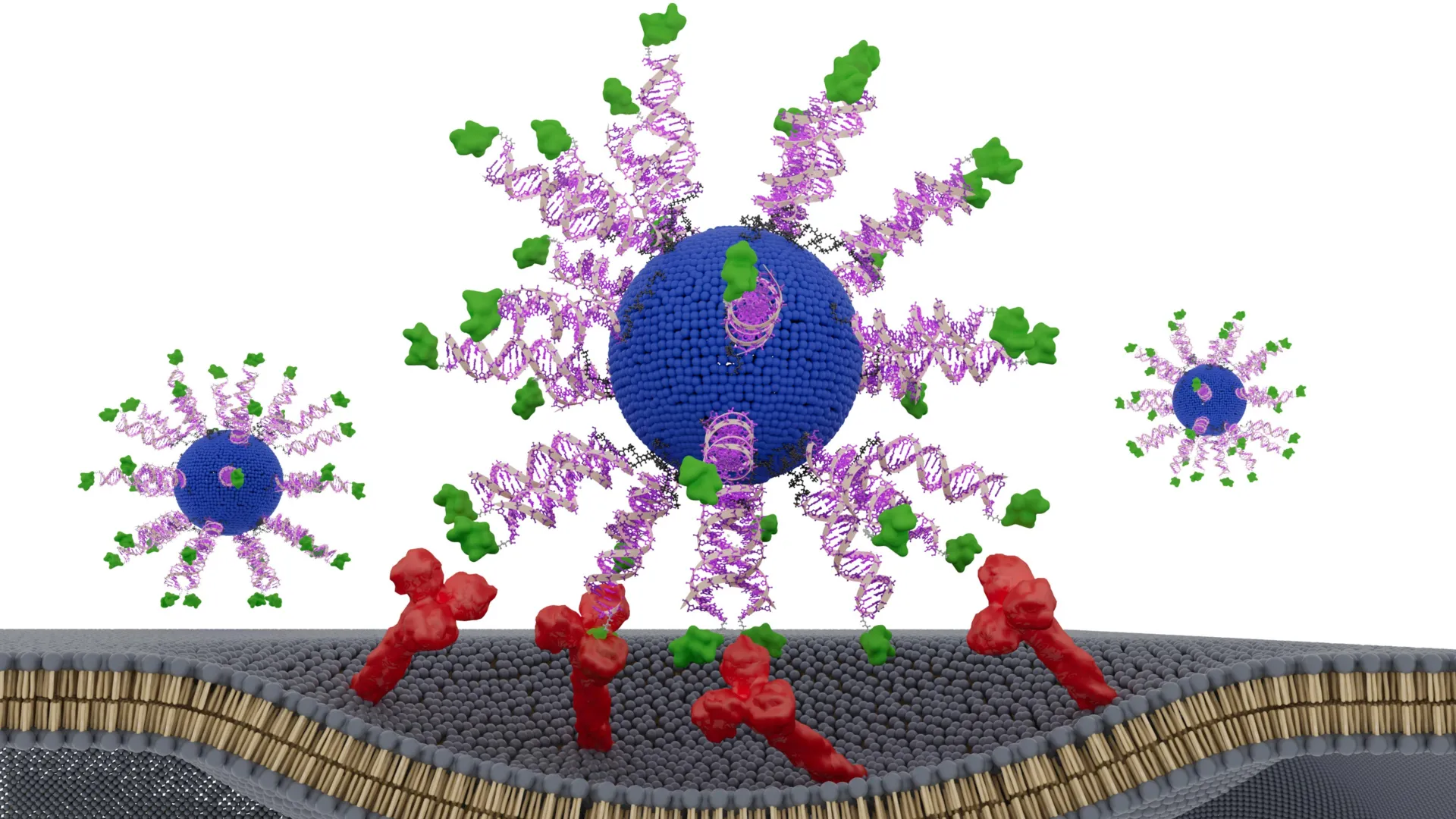
To rigorously investigate this hypothesis, the research team engineered a novel vaccine built upon the foundation of a Spherical Nucleic Acid (SNA). SNAs are revolutionary globular structures composed of DNA or RNA that possess a unique ability to naturally and efficiently enter immune cells, thereby initiating and orchestrating powerful immune responses. This inherent cellular uptake mechanism makes SNAs an ideal platform for delivering therapeutic payloads directly to the cellular machinery responsible for immune activation.
The innovative aspect of this study involved intentionally manipulating the components within the SNA framework, creating several distinct spatial configurations. Each meticulously designed version of the vaccine was then subjected to comprehensive evaluation. This included testing in sophisticated humanized animal models specifically engineered to mimic HPV-positive cancers, as well as ex vivo analyses using actual tumor samples meticulously collected from patients suffering from head and neck cancer. This dual approach ensured that the findings had both a robust preclinical basis and direct relevance to human disease.
Through this exhaustive evaluation, one particular SNA configuration emerged as unequivocally superior. This optimized arrangement not only significantly curtailed tumor growth and markedly prolonged survival rates in the animal models, but also generated substantially greater numbers of highly active and tumor-specific CD8+ T cells—the immune system’s most formidable "killer" cells against cancer. These compelling results unequivocally demonstrate that even minor, nanoscale alterations in the spatial arrangement of vaccine components can be the decisive factor determining whether a nanovaccine elicits a negligible immune response or unleashes a robust, tumor-destroying therapeutic effect.
This foundational principle is the cornerstone of an burgeoning scientific discipline known as "structural nanomedicine." This term, originally coined by Northwestern’s distinguished nanotechnology pioneer Chad A. Mirkin, encapsulates a scientific philosophy centered on the precise engineering of nanoscale structures for medical applications. The field heavily leverages SNAs, which Mirkin himself invented, as a versatile and powerful toolkit for designing next-generation therapeutics and diagnostics.
"There are thousands of variables in the large, complex medicines that define vaccines," articulated Mirkin, who served as the lead investigator for this landmark study. His statement highlights the inherent complexity of traditional vaccine design, often involving myriad components with poorly defined interactions. "The promise of structural nanomedicine is being able to identify from the myriad possibilities the configurations that lead to the greatest efficacy and least toxicity. In other words, we can build better medicines from the bottom up." This "bottom-up" approach represents a radical departure from conventional methods, promising an era of rationally designed, highly optimized medical interventions.
Professor Mirkin holds an impressive array of appointments at Northwestern University, reflecting the interdisciplinary nature of his work. He is the George B. Rathmann Professor of Chemistry, Chemical and Biological Engineering, Biomedical Engineering, Materials Science and Engineering, and Medicine, spanning the Weinberg College of Arts and Sciences, McCormick School of Engineering, and Northwestern University Feinberg School of Medicine. Furthermore, he directs the internationally renowned International Institute of Nanotechnology and is a key member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Mirkin co-led this pivotal study with Dr. Jochen Lorch, a distinguished professor of medicine at Feinberg and the medical oncology director of the Head and Neck Cancer Program at Northwestern Medicine, bringing crucial clinical expertise to the research.
Transcending the Limitations of Traditional "Blender Approach" Vaccines
Conventional vaccine development has historically relied on a more empirical and less precise methodology. It often entails simply combining essential active ingredients, such as tumor-derived molecules known as antigens and immune-stimulating compounds termed adjuvants, into a single formulation. This mixture is then administered with the hope of generating an effective immune response.
Mirkin vividly describes this conventional method as the "blender approach," a metaphor that perfectly illustrates the lack of precise structural control and defined organization among the components. The underlying assumption has often been that the biological system will sort out the components and present them effectively to the immune system, an assumption that structural nanomedicine now challenges.
"If you look at how drugs have evolved over the last few decades, we have gone from well-defined small molecules to more complex but less structured medicines," Mirkin elaborated, providing historical context for the current paradigm. He cites the rapid development of the COVID-19 vaccines as a prime example: "The COVID-19 vaccines are a beautiful example — no two particles are the same. While very impressive and extremely useful, we can do better, and, to create the most effective cancer vaccines, we will have to." While undeniably effective in addressing a global health crisis, the inherent heterogeneity and lack of precise structural control in these complex formulations suggest room for improvement, particularly when aiming for highly specific and potent anti-cancer responses.
Research emanating from Mirkin’s laboratory consistently demonstrates that by meticulously arranging antigens and adjuvants into precisely designed nanoscale architectures, outcomes can be dramatically enhanced. When these components are properly configured within an SNA, the same ingredients can yield significantly stronger therapeutic effects with a reduced profile of toxicity compared to their unstructured, "blender" counterparts. This ability to achieve superior results without increasing the dose or introducing new, potentially toxic compounds represents a major advantage for patient safety and treatment efficacy.
The versatility of this structural nanomedicine strategy is already evident in its broad application. Mirkin’s team has successfully utilized this approach to design SNA vaccines targeting a diverse range of aggressive cancers, including melanoma, triple-negative breast cancer, colon cancer, prostate cancer, and Merkel cell carcinoma. These investigational candidates have consistently shown highly encouraging results in rigorous preclinical studies, paving the way for further clinical translation. Indeed, the real-world impact of SNAs is already tangible, with seven SNA-based drugs having successfully advanced into human clinical trials for various diseases, demonstrating their safety and potential efficacy. Beyond therapeutics, SNAs are also incorporated into more than 1,000 commercial products, underscoring their broad utility across scientific and industrial sectors.
Amplifying CD8 T Cell Response Against HPV Cancers with Precision
In this latest study, the researchers strategically focused their efforts on cancers etiologically linked to the human papillomavirus (HPV). HPV is a well-established causative agent for the vast majority of cervical cancers globally and is increasingly implicated in a growing percentage of head and neck cancers, posing a significant public health challenge. While highly effective preventive HPV vaccines exist and have proven instrumental in averting initial infection, they are not designed to treat cancers that have already developed. This unmet medical need creates a critical therapeutic gap that the Northwestern team aimed to address.
To meet this challenge, the team engineered novel therapeutic vaccines explicitly designed to activate CD8 "killer" T cells. These cytotoxic T lymphocytes are the immune system’s most potent weapon against cancer cells, capable of directly recognizing and destroying malignant targets. Each nanoparticle within the vaccine construct was composed of a lipid core, a sophisticated immune-activating DNA sequence, and a short, immunogenic fragment of an HPV protein that is characteristically expressed in tumor cells.
Crucially, every version of the vaccine created for this study contained an identical complement of ingredients. The sole variable was the precise position and orientation of the HPV-derived peptide, or antigen, within the SNA structure. The researchers meticulously tested three distinct designs. In one configuration, the peptide was deliberately concealed within the core of the nanoparticle, potentially limiting its accessibility to immune cells. In the other two designs, the peptide was prominently displayed on the surface of the SNA. For these surface-displayed versions, a subtle but potentially impactful distinction was introduced: the peptide was attached at either its N-terminus or its C-terminus. This seemingly minor chemical difference can profoundly influence how immune cells recognize, bind, and process the antigen, thereby shaping the ensuing immune response.
The experimental results were striking and definitive. The version of the vaccine that presented the antigen on the SNA surface, specifically attached via its N-terminus, consistently produced the most robust and potent immune reaction. This optimized configuration triggered up to an eightfold increase in the production of interferon-gamma, a critical anti-tumor cytokine released by activated killer T cells, signaling their cytotoxic activity. Furthermore, these T cells, generated by the N-terminus displayed antigen, exhibited substantially greater efficacy in destroying HPV-positive cancer cells in vitro. In the sophisticated humanized mouse models, tumor growth was markedly slowed, and survival rates were significantly improved. Perhaps most compellingly, in ex vivo analyses of actual tumor samples harvested from HPV-positive cancer patients, the ability of immune cells to kill cancer cells increased by a remarkable twofold to threefold.
"This effect did not come from adding new ingredients or increasing the dose," emphasized Dr. Jochen Lorch, highlighting the elegance and efficiency of the discovery. "It came from presenting the same components in a smarter way. The immune system is sensitive to the geometry of molecules. By optimizing how we attach the antigen to the SNA, the immune cells processed it more efficiently." This statement underscores the profound biological principle at play: the immune system is a sophisticated recognition machine, and the precise presentation of antigens is paramount for optimal engagement.
Redesigning Cancer Vaccines with Unprecedented Precision and the Aid of AI
Armed with this groundbreaking understanding, Professor Mirkin now envisions a future where past vaccine candidates, which may have shown initial promise but ultimately failed to elicit sufficiently strong immune responses in clinical trials, can be revisited and re-engineered. By definitively demonstrating that nanoscale structure directly dictates immune potency, this research provides a powerful, actionable framework for significantly improving therapeutic cancer vaccines using existing, well-characterized components. This strategic approach holds immense promise for accelerating vaccine development timelines and substantially reducing the exorbitant costs typically associated with bringing new drugs to market, as it leverages known entities rather than requiring the discovery of entirely new molecules.
Looking further ahead, Mirkin anticipates that artificial intelligence (AI) and machine learning will become indispensable tools in the arsenal of vaccine design. These sophisticated computational systems possess the capacity to rapidly analyze and model an astronomical number of structural combinations, far beyond what human researchers could manually explore. By identifying the most effective arrangements with unprecedented speed and accuracy, AI could revolutionize the drug discovery pipeline, transforming it from a largely empirical process to a highly predictive and optimized one.
"This approach is poised to change the way we formulate vaccines," Mirkin declared with conviction, emphasizing the transformative potential of structural nanomedicine. "We may have passed up perfectly acceptable vaccine components simply because they were in the wrong configurations. We can go back to those and restructure and transform them into potent medicines. The whole concept of structural nanomedicines is a major train roaring down the tracks. We have shown that structure matters — consistently and without exception." His statement is a call to action, signaling a new era of rational design in medicine where precision at the nanoscale unlocks unprecedented therapeutic efficacy.
The study, meticulously detailed in the paper titled "E711-19 placement and orientation dictate CD8+ T cell response in structurally defined spherical nucleic acid vaccines," received critical financial backing from several esteemed organizations. These included the National Cancer Institute (under award numbers R01CA257926 and R01CA275430), the generous support of the Lefkofsky Family Foundation, and contributions from the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, all of which underscore the significance and potential impact of this pioneering research.